A Ceramic Materials Atlas

Mining Raw Barite near Sophia Bulgaria [courtesy of RUA Mining Group Website https://rua-group.com]
Share:
It seems obvious, but all the materials we use to make any work of art come from the surface of the earth. For about 27,000 years humans have been making objects and artworks from clay. Every one of these objects, along with everything around us—our own bodies, the images on this screen, our thoughts, our desires, our ethical actions—are all part of the dynamic material body of our planet.
Over the last five years I have been working with a group of colleagues on a project called the Ceramic Materials Atlas. This project began with a straightforward idea to track all the materials we use in the pottery studio where I teach ceramics and sculpture at Colorado State University back to their origins in the earth. The materials used within the studio ceramic process are among the most prevalent in the earth’s lithosphere, and they exist in nearly every region. In the not-so-distant past, potters would have dug their clay and minerals for glazes from an area within a few miles of their studios. Although this practice continues today, the vast majority of potters and artists who use clay now source their materials from multinational corporations with ties to mining sites around the globe rather than from local stream beds. Materials used in ceramics are just one example underscoring how our modern lives are entangled with systems of industrial mineral extraction.
Blue Marble, 1972, photograph from Apollo 17 [courtesy of NASA]
In a typical pottery studio there are about 50 materials. The ceramic materials at Colorado State University are more or less similar to ones in countless other studios throughout North America. The ingredients arrive at the studio in 50 pound bags of homogeneous white or beige powder, stacked on pallets, from which they may then be transferred into bins and jars in the glaze lab. Organizing these materials in our studio is the outermost tip of a complex global distribution network of intricate webs of highways, shipping routes, rail lines, and flight paths—a planetary vascular system and neural network, a pulsing flow of matter and information covering the surface of the earth.
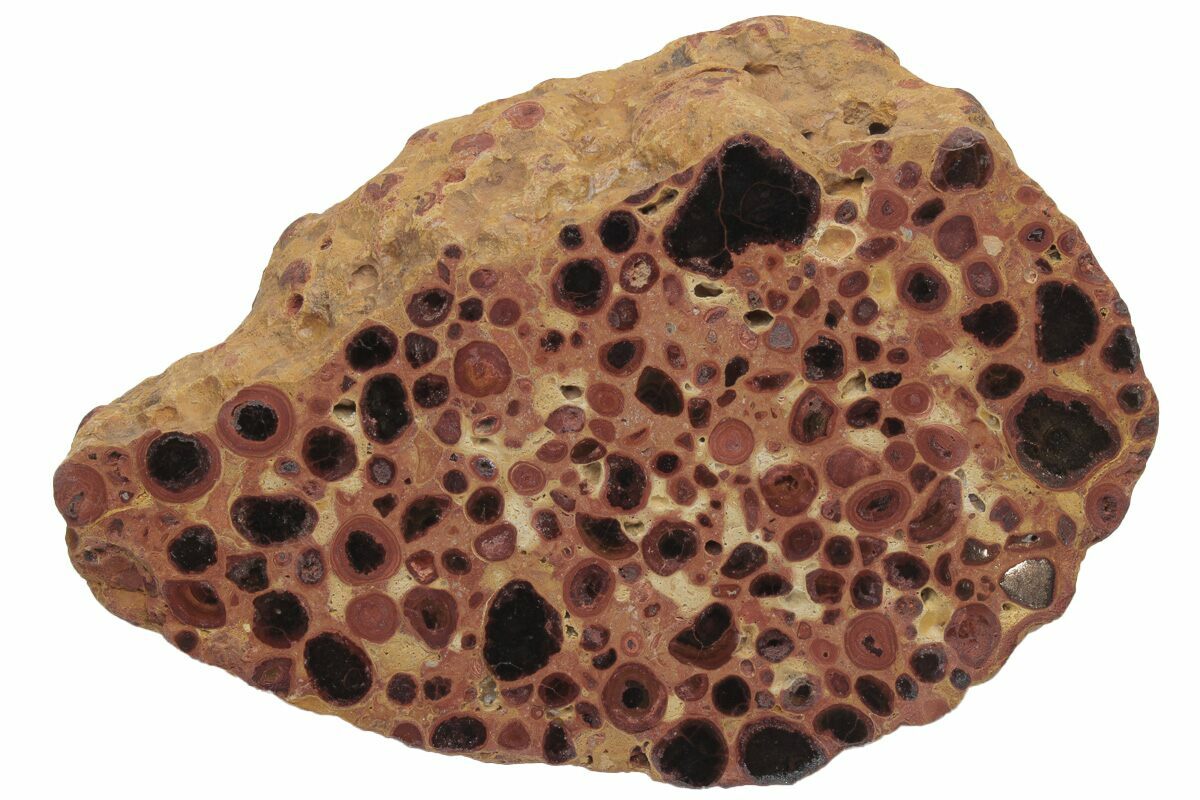
Barite Crystal on a Dolomite Matrix [photo: Ivar Leidus; courtesy of Creative Commons]
These materials are pulled from the ground by machines of incomprehensible size and power that sculpt the surface of the planet, carving and piling anthropogenic canyons and mountains. Today, mining operations move more material on Earth’s surface than is moved by all weather, wind, rivers, oceans, and geologic processes combined. These human-made mountains are sorted, filtered, sifted, and refined, particle by particle, then molecule by molecule, down to nanoscale, to arrive at an ethereal essence. Uncountable particles of distilled matter are then packed into brown and white bags and shipped to ceramic studios worldwide.
Barium carbonate offers one example of this journey of extraction and hyper-refinement. The provenance of this material leads us along a circuitous path to the edge of a vast, human-made hole in Bulgaria. Known as the Kremikovski Pit, this mine is big as a canyon—the result of decades of iron ore extraction for one of the largest steel refineries in Europe. Here, just below the lip of the gaping pit, barite formed in the earth alongside iron, through the geologic process of hydrothermal metasomatic replacement. This process, as one mineral is exchanged for another—much like the way wood petrifies as its organic minerals are replaced with silica—occurs over tens of thousands of years. Deep in the ground, the process is accelerated as rising pockets of magma heat the mineral-rich groundwater.
The extraction of barium from barite, its ore, is a complex and energy-intensive process that includes grinding, filtering, and the use of giant magnets. Once this process is complete, a reduced quantity of barite is shipped from a port a few hundred miles from the Kremikovski mine to Germany. Then, it’s loaded onto trucks and driven to an immaculate factory in the town of Bad Hönningen. There, through an intricate chemical process, they produce a “99% pure” grade of barium carbonate, which eventually makes its way to thousands of ceramic studios as a distilled, white, nondescript powder.
At the studio, this powder can be remixed to produce seemingly endless derivations of clays and glazes. When added to glazes in specific amounts, barium carbonate produces a subtle matte texture, the result of microscopic crystals growing within the glaze as it cools. Throughout history, in their pursuit of ever-more novel, resilient, and predictable results, ceramic artists have engaged in a long tradition of knowledge production about the earth’s mineral skin.
This knowledge enfolds again as our human-geologic ceramic mixtures are then heated within kilns. Like all “human” heat sources, kilns are simply tools for concentrating energy that dissipates from our cooling planet and from our nearest burning star. The energy focused within the kiln creates a small pocket of transformative heat—a geologic process all its own—that controls the melting and cooling of pots and sculptures. Intricate Anthropocene crystals.
This terrifying network of industrial extraction is either hidden or inescapably present in the daily existence of any individual human life, and it tracks alarmingly with patterns of human inequality and ongoing histories of colonial extraction. Like so many materials that artists use in the studio, alumina hydrate exemplifies this ongoing reality.
In ceramic glazes alumina can produce a soft pink color, like the inner ear of a baby rabbit. Alumina is also highly resistant to melting, even at extreme heat. This resistance makes it useful in the production of refractory materials such as heat-resistant bricks, and washes that protect kiln interiors from corrosion. The Rio Tinto Alcan refinery located on the Saguenay River, outside Montreal, Quebec, produces the small amount of alumina hydrate that we use in the studio, along with tons and tons of raw aluminum shipped throughout North America for the manufacturing of airplanes, cans, cars, and a broad range of other objects. In this enormous factory—the size of a small city—alumina is refined from the raw mineral bauxite.
There are many bauxite mines around the world, but this Canadian factory most often receives materials shipped via giant ocean freighters from the small town of Porto Trombetas, deep in the Brazilian Amazon. Bauxite forms in that tropical climate because of alumina’s resistance to weathering. It concentrates in the soil over thousands of years as surrounding minerals slowly erode away.
Bauxite Mining panorama in Trombetas Brazil [courtesy Engineering and Mining Journal]
Paragominas Bauxite mine in Pará, Brazil [courtesy of Hydro]
Polished Bauxite (Aluminum Ore) [courtesy of FossilEra]
To extract bauxite from the ground, the global mining company Mineração Rio do Norte removes the entire rainforest canopy, rolling it up like a carpet. Although the company claims an elaborate technique for replacing vegetation, the process results in an obvious and irreparable loss of rainforest biodiversity. What’s more, as bauxite is refined, the remaining soil is washed into the local watershed where it decimates fisheries that the Quilombola, and other regional Indigenous populations, depend upon for sustenance.
Alumina hydrate thus becomes intertwined with the violence of global industrial extraction. Yet, as with all materials entering studio ceramics, it represents a minuscule, functionally insignificant portion of that material’s use. In other words, even if every ceramic artist in the world stopped using alumina hydrate—or cobalt, or lithium—the regime of global extraction of such materials would continue, entirely unaffected. I mention this reality, not to absolve any ethical responsibility for the extent to which ceramics—like so many aspects of our lives—are intertwined with the violence of industrial extraction. Instead, the Ceramic Materials Atlas aspires to the ecological ethic proposed by political theorist Jane Bennett and philosopher Timothy Morton. For them, an ethical engagement with the material world grows from a cultivation of attention toward the fullness and complexity of material realities. This engagement with material ontology does not end with information gathering, accounting for carbon footprints, or labeling materials as “green.” Rather, it is an ongoing practice of cultivated wonder and careful attention toward the complicated beauty of materials, the violent realities of material extraction, and all other delicate moments in which material beings shimmer at the cusp of our apprehension.
One of the most commonly used clays in ceramic studios is ball clay. The name comes from its characteristic sticky texture, or plasticity, which allowed early miners to roll the material out of the ground in balls. There are several common ball clays in North America, most of which come from an area along the border between Kentucky and Tennessee. Today, there are four active clay mining companies in this area, both located near the town of Paris, Tennessee.
Ball clays were deposited here about 50 million years ago, during the hot and humid climate of the Eocene. During that epoch, crocodiles lived on the land that is now the south pole, and there was no permanent ice on the planet. Thanks to much higher global sea levels, the region that today encompasses Tennessee and Kentucky rested along the expanded coast of what is now called the Gulf of Mexico. There, after a long journey downhill from the Appalachian Mountains, clays were deposited in tidal pools. That clay began its life even earlier, about a billion years ago, as a set of aluminate and silicate minerals held inside granite. Given long enough timelines, the earth’s surface proves fluid. Granites form when the surface is pushed downward, into the hot mantle. There, lithosphere minerals melt and reform into granite. Over millions of years, the granite is eventually pushed back toward the surface, where contact with the atmosphere cools it, and where it takes on the stoney surface we know.
Jay Matternes, Wyoming Rainforest, Early to Middle Eocine (50.3 to 46.2 million years ago), 1960, [courtesy of Smithsonian National Museum of Natural History]
As with global warming today, the hot Eocene climate was caused by a carbon-rich atmosphere that trapped heat from the sun. As rain fell, it mixed with carbon in the atmosphere and became mildly acidic. That acid rain, falling on the Appalachians, slowly decomposed the granite and softened the sharp contours of those ancient mountains. As granites decompose, their alumina and silica combine with water in the atmosphere to create a new mineral called kaolinite, the primary mineral in ball clay. Ball clays are the dust of ancient mountains.
Scanning Electron Microscope image of Kaolinite [photo: Johannes T. Kehren; courtesy of Hochschule Koblenz; JEOL JSM 7200F SEM]
Under a microscope, kaolinite consists of tiny hexagonal particles. The surfaces of these plate-like particles attract water. When force is applied by, say, the pressure of your fingers, the particles slide against each other and lock into place. More than 25,000 years of pottery has issued from this quality of plasticity—the essence of clay.
Like clay, our own bodies are made from water, carbon, and minerals from the surface of the earth. Like clay, we are made from the dust of ancient mountains. The word human shares an etymological root with humus, meaning soil or earth. As we shape clay, it also shapes us in a deep, material sympathy. To paraphrase sociologist Richard Sennett, we think through making and with materials. Clay is not an inert external substance—not merely a tool to be used. Instead, as we make things, our hands and the clay we are shaping merge with the mind. The mind thereby makes the mind—and making itself is a work of continuous becoming. The pot would not be formed into a thin shell of a vessel without its interaction with the intelligence of a human, material body. But neither would a human create this shape, if not for the interaction with the intelligence held within the material body of the clay. In this sense, the human beings around you are like the clay you might hold in your hands—both parts of the material intelligence of our planet, unfolding in geologic time.
The entire system of material interactions that contribute to a ceramic object’s formation—the movement of Earth’s surface, unfolding in deep time; the planet-scale network for distribution and sorting of matter; the mineral and chemical interactions within our own bodies that form our thoughts; the experience of beauty, and our yearning to participate in shaping the body of the earth and this creation of meaningful form—are all geologic processes. Our thoughts are also geologic forms—ones made by the movement of our planet, which is itself delicate and ephemeral. For an instant, each crystalline thought glimmers on the surface of our perception, only to disappear and descend back into the unknowable fullness of the mineral earth.
Del Harrow lives and works in Fort Collins, CO with his wife, the Potter Sanam Emami and their son, William. Harrow is a Professor at Colorado State University where he teaches Sculpture, Digital Fabrication, and Ceramics. His Sculpture and Ceramics integrate traditional manual and skill based forming processes with digital fabrication technology. His work is represented by Haw Contemporary in Kansas City, MO, and Harvey Preston gallery in Aspen, CO and has been supported by grants from the North Carolina Center for Craft, the Windgate Foundation, The Graham Foundation, and a 2020 United States Artists Fellowship.